About Us
ELMED was established in 1991 by engineers and urology doctors to produce Turkey’s FIRST lithotripsy Systems for kidney stone management. ELMED, which started the production of high-tech medical devices in Ostim – Ankara, has been continuing its activities as a leading manufacturer in the field of urology for 30 years.
According to the market research, conducted by independent organizations, ELMED has become a worldwide known brand among the World’s top ten companies in the lithotripsy market.
In many current urology books and medical publications, information is given about the devices that we have developed and produced, and special sections are written.
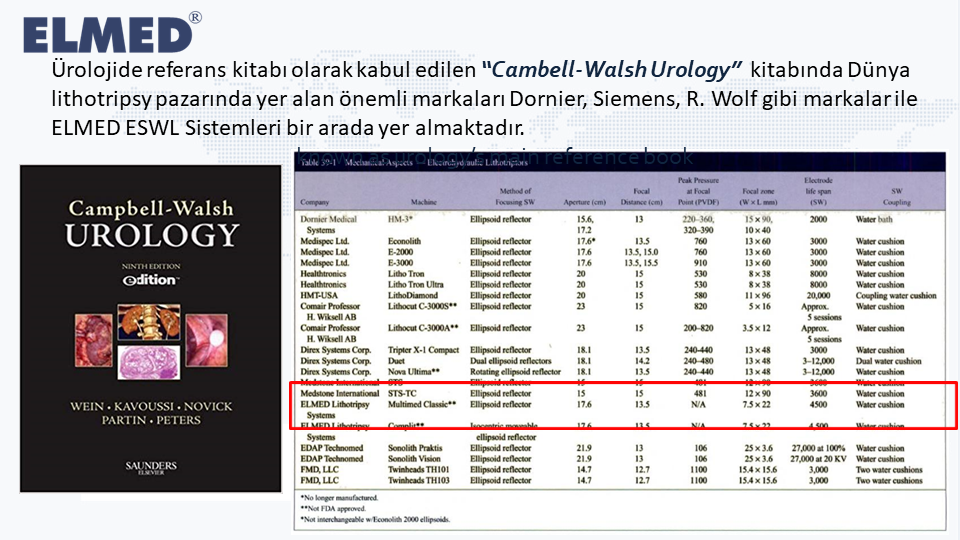
In the book “Cambell Walsh Urology”, which is accepted as a reference book in urology, important brands such as Dornier, Siemens, R. Wolf in the world lithotripsy market and ELMED ESWL Systems are included.
ELMED first exported in 1994. Our products, which have international quality certificates and special permits of countries, have been installed by exporting to more than 60 countries.
Our company, which specializes in the treatment of urinary system stones, produces Extracorporeal Kidney Stone Breaking Systems (ESWL: Extracorporeal Shock Wave Lithotripsy) and Intracorporeal Pneumatic Lithotripsy as its main product group.
ELMED designed and produced the World’s unique robot Avicenna Roboflex for flexible ureteroscopic laser lithotripsy within the scope of the government-supported project.
Research and Development
Increasing its production capacity based on R&D, ELMED continues to develop high value-added products in its modern production facility with a closed area of 1,800 m2 within Ostim Technopark, with the financing support provided by the government for R&D companies. ELMED adds value to the country’s economy with its productions.
Our Quality Policy
ELMED , takes the required technological steps in all stages of the system starting with the purchase.
For a continuously improved medical performance, we follow modern business management principles to ensure the safety and reliability of our products and to meet the expectations of healthcare workers, patients and the society. This includes the implementation of a quality management system based on EN ISO 13485:2016 and EN ISO 9001:2015 standards, thus meeting existing legal requirements as well as those set by the authorities. In other words, our quality policy ensures compatibility between our products and the legal provisions in our target markets. The Medical Device Directives (93/42/EEC and 2007/47/EC) in the EU market drive our understanding of quality.
Our system covers all quality-related processes throughout the company.
Our quality policy is driven by the basic principles outlined below:
- Commited management in order to satisfy the requirements of the quality system and maintain its effectiveness .
- Ensuring disciplined and hierarchical participation by all our employees in the production process, with a continuously increasingquality awareness
- Understanding customer needs with the help of ongoing communication, which helps us meet and surpass expectations and continuously improve our performance and competence
- Creating and increasing quality awareness in our suppliers
- Complying with international production brand name in domestic and international markets
- Continuously monitoring our quality policy and implementation methods to meet changing market expectations and ensure continuity